In the Great Puzzle of decarbonization, green hydrogen points ways to become one of the most important pieces. It has become one of the great bets of the European Union for the energy transition, but although renewable energies such as solar or wind are used to produce it, it has a big problem: it consumes a huge amount of fresh water. Some researchers want to stop this problem using something we produce in industrial quantities.
Sewage.
The water problem. Talking about clean energy implies looking at some initial point of the process to realize that there is still an ecological footprint. Electric cars do not emit, but manufacture their batteries yes, for example. Something similar happens with green hydrogen. Solar or wind energy is what is used to perform the electrolysis process with which hydrogen is generated to use as an energy source, but as we said, a lot of water is consumed, a resource that is increasingly scarce for millions of people.
That is why we are investigating alternative ways to generate green hydrogen without those huge amounts of fresh water. For example, using seawater, but there is a type of fresh water that had not been considered for the process and has now entered the equation.
Waste Treasury in wastewater. These waters contain a series of pollutants that, according to logic, would hinder the electrolysis process. They have nickel, platinum, chromium and other metals that, until now, had to be extracted from water in an expensive purification process before using that water in electrolysis. However, a team from the School of Sciences of the Australian Rmit has found a way to take advantage of these metals to accelerate the production of green hydrogen.
In electrolysis, electrodes are a key component because it is the one that facilitates the reaction that separates water in its base components: hydrogen and oxygen. To do this, an anode is used (where water breaks down releasing oxygen and electrons) and a cathode (protons earn electrons and form hydrogen molecules). In the anode and cathode metals such as nickel, platinum or iride are used as those found in wastewater, and what they have done from the RMIT is … take advantage of them.


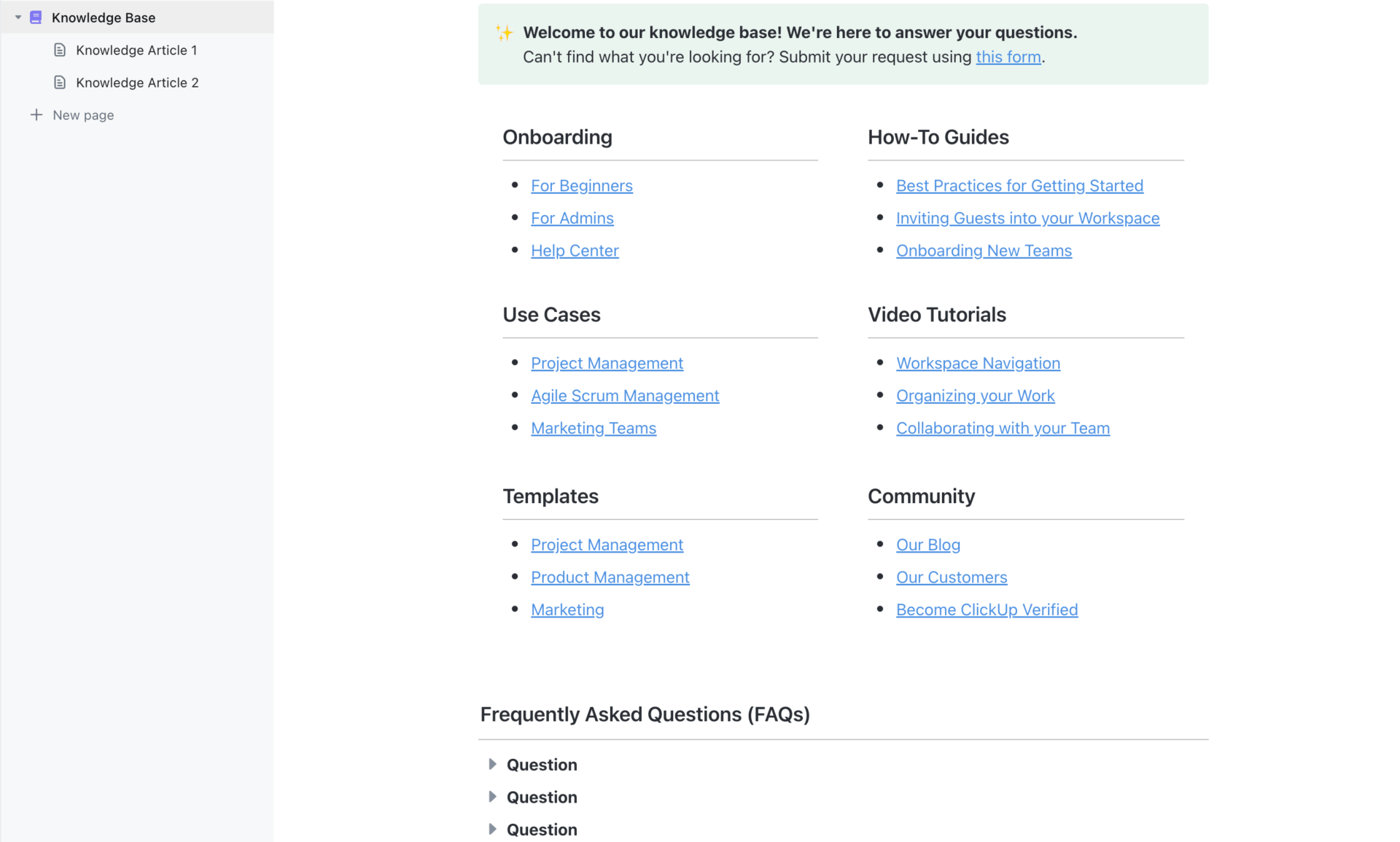
The invention. To do this, the electrode is manufactured with an absorbent carbon surface that attracts those metals present in wastewater, as if it were a magnet. When they “catch them”, form catalysts that conduct electricity and start that task of dividing water into its components.
Nasir Mahmood is one of the researchers and, as we read in MirageNews, explains the reaction as follows: “The catalyst accelerates a chemical reaction without consuming in the process, allowing metals to interact with other elements present in wastewater and enhancing the necessary electrochemical reactions to divide oxygen and hydrogen water.
And, beyond the theory, the team devised a device that managed to operate continuously for 18 days with a minimal decrease in performance and achieving 89% efficiency in energy conversion and, as stated in ACS, a stability of 95%. This pilot device, connected to a small solar plate, is the one you can see in the image that opens this article. And the waters look at everything … except purified water.
Potential. Now, it is not as easy as taking the wastewater and using it directly. The team confirms that it used wastewater that had been subjected to some treatment to eliminate solid waste, organic matter and other nutrients. Not metals, yes.
The water used for the experiment comes from agricultural waste, which opens another door to the circular economy of the materials. It is estimated that more than 80% of wastewater returns to the planet without any treatment (although other sources point to 50%), but if we start using a part to produce green hydrogen, we would be reducing that percentage, giving a break to areas with drought problems and allowing to inject energy into those areas without affecting their drinking water deposits. In developing countries it would have great potential.

An upcoming step is to try more types of wastewater, since not all have the same amount of metals in their composition, and as professor Nicky Eshtiaghi, another of the authors of the study, comments, the plan now is to look for partners to climb technology and find commercial applications.
Images | Rmit, hightail
WorldOfSoftware | In Peru, a company has had an idea to take wind energy directly to your home: turbines as a lay way