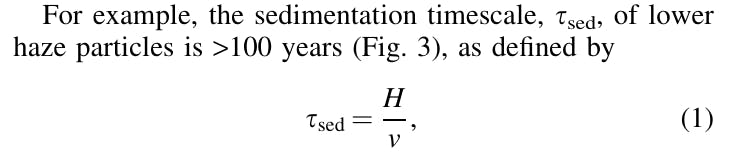Authors:
(1) Sara Seager, Departments of Earth, Atmospheric and Planetary Sciences, Physics, Aeronautics and Astronautics, Massachusetts Institute of Technology, Cambridge, Massachusetts, USA;
(2) Janusz J. Petkowski, Department of Earth;
(3) Peter Gao, Department of Astronomy, University of California at Berkeley, California, USA;
(4) William Bains, Department of Earth;
(5) Noelle C. Bryan, Department of Earth;
(6) Sukrit Ranjan, Department of Earth;
(7) Jane Greaves, School of Physics and Astronomy, Cardiff University, Cardiff, United Kingdom and Institute of Astronomy, Cambridge University, Cambridge, United Kingdom.
Table of Links
Abstract and 1. Introduction and Overview
- Challenges and Assumptions for Life in the Venusian Clouds
- A Proposed Cycle for Venusian Aerial Microbial Life
- Discussion
- Summary, Acknowledgments, Author Disclosure Statement, Funding Information, and References
3. A Proposed Cycle for Venusian Aerial Microbial Life
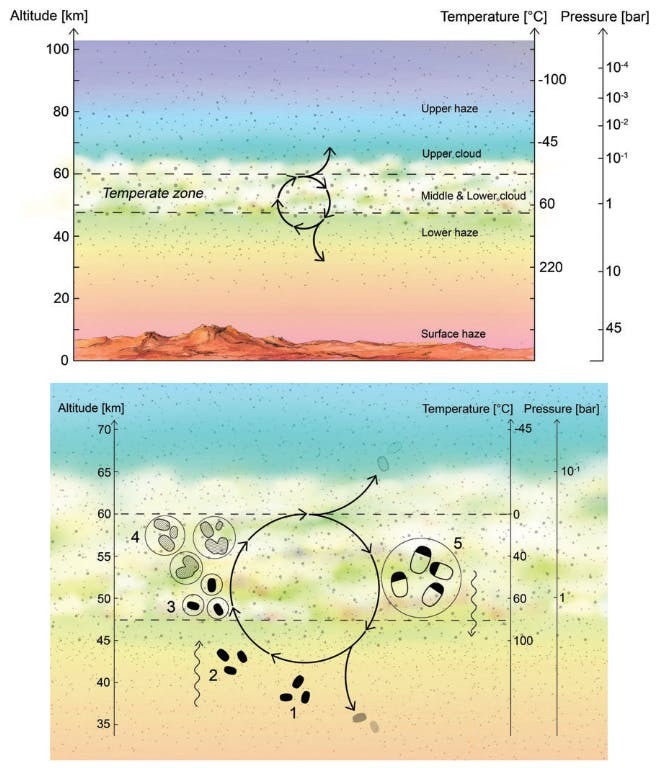
We propose a life cycle for Venusian microbes (Fig. 1) that begins in the lower haze layer where desiccated spores reside in a dormant phase. The spores are transported upward by vertical mixing induced by gravity waves to a habitable layer of temperate conditions. Acting as CCN, the spores become encased in a liquid droplet (mostly H2SO4 with some H2O) and germinate. During months aloft, the bacteria metabolize and divide. The cloud droplets, meanwhile, collide and grow to a size large enough that gravity forces them to settle downward. On the downward journey, triggered by changes in the environment (increasing temperatures and the concomitant evaporation of liquids), the bacteria sporulate, preserving themselves as desiccated spores. Once reaching the stable, long-lived stagnant lower haze layer ‘‘depot,’’ the spores remain dormant until the life cycle can begin again. In this section, we describe each step in more detail.
3.1. Step 1: Desiccated spores populate the lower Venus atmosphere haze layer, a depot of hibernating microbial life
Venus has a lower haze layer of relatively low mass and unknown composition (Titov et al., 2018), such that desiccated bacterial spores could be a minor component. The lower haze layer resides below Venus’ lowest clouds, at altitudes of 47.5 km down to 33 km, where temperatures range from 350 K to 460 K (Zasova et al., 2006). Under pressures of around 10 bars (Taylor and Hunten, 2014), these temperatures are too high for water to exist in liquid form and are also high enough for sulfuric acid to thermally decompose (Knollenberg and Hunten, 1979; Knollenberg et al., 1980; Krasnopolsky, 2013; Titov et al., 2018). What is known about the haze layer is the particle size and number density (Fig. 2), derived from Pioneer Venus data reported in the canonical work by Knollenberg and Hunten (1980).
Desiccated spore sizes must be consistent with the small particle radii in the lower haze layer. This motivates the question of whether spores of that small size are large enough to contain all the required ‘‘cell machinery.’’ Although the question of minimum cell size has only been considered for a hydrated free-living cell, it is likely similar for a spore. Cells must have sufficient space to accommodate metabolic machinery. Based on terrestrial life, a cell is unlikely to be much smaller than 0.2 mm in diameter based on the actual volume of genetic material, enzymatic complexes required for replication, transcription, and translation, in addition to a set of other proteins that contribute to basic physiological processes (Luef et al., 2015; Chen et al., 2018).
Although these minimal cell sizes are established for Earth’s microbial life, it is unlikely that the hypothetical Venusian aerial life can be much smaller. Any complex metabolic activities would require complex biochemical machinery, which would require enough cell volume to function properly, even if the chemical basis of Venusian life is different than on Earth. Interestingly, the minimal recorded bacterial spore size on Earth is 0.25 mm diameter (Staley, 1999), with a more general range of 0.8 to 1.2 mm diameter (Krieg and Holt, 1984; Ricca and Cutting, 2003). For comparison, mean particle radii in Venus’ lower haze layer generally range from 0.2 to 0.5 mm, but with radii as large as 2 mm at the top of the lower haze layer (Knollenberg and Hunten, 1980) (Fig. 2). Therefore, the particle size distribution in the Venusian atmosphere lower haze layer is compatible with the known range of cell and spore sizes of Earth’s microorganisms.
We refer to the lower haze layer as a depot due to its relative stagnation compared with the rest of the Venus atmosphere, allowing desiccated bacteria to persist for a prolonged period.
where H is the scale height (on the order of 5 km; Seiff et al., 1985) and v is the sedimentation velocity (Fig. 3). In addition, the lower haze layer is stable against convection, meaning that vertical transport by convection does not occur. The stable stratification inference is based on in situ lapse rate measurements by the Pioneer Venus probes (Schubert et al., 1980). Therefore, there is no convective overturning to transport haze particles upward, or downward to the hot, deep atmosphere (Schubert et al., 1980).
Venus has a Hadley cell flow that moves upward at the equator and downward at the poles, but not enough is understood to know what altitude particles trapped in the flow may be lofted to or deposited in. In addition, the location of the returning branch is not known and thus may not impact the lower haze (Sa´nchez-Lavega et al., 2017).
3.2. Step 2: Desiccated spores in the lower Venus atmosphere haze layer travel up to the lower clouds by mixing via gravity waves, followed by convective entrainment
The spores in the lower haze layer must be transported upward to continue their life cycle, but the relative stagnation of the lower haze layer, as described in Section 3.1, creates a challenge. One possible solution is the action of gravity waves, which appear to be present in the lower haze layer due to the layer’s static stability. Although gravity waves can only lead to the net transport of energy and momentum and not matter, they can compress atmosphere layers as they travel and contribute to atmospheric mixing.
Gravity waves operate in both vertical directions, meaning that some desiccated spores will be lost to lower, hotter layers. Thus, the lower haze layer may be more of a ‘‘leaky’’ depot for desiccated spores.
In summary, although the dynamics of the lower haze layer are highly uncertain, upward (and downward) transport of haze particles is likely accomplished through mixing via gravity waves. Once transported upward to the bottom of the lower cloud layer, particles may continue to efficiently move upward into the clouds by convective entrainment.
3.3. Step 3: The desiccated spores act as CCN and once surrounded by a liquid droplet germinate to a metabolically active life-form
We have argued in Section 2.1 that active microbial life must live inside a droplet, so the spores must be only part of the life cycle. This section describes how the spores become engulfed in cloud droplets.
Once transported to the Venus lower cloud layer, the spores must act as CCN. CCN are ‘‘cloud seeds,’’ a small solid surface needed for vapor to condense. Unlike the relatively high temperature at lower haze altitudes, the lower cloud layer has temperatures where liquid and vapor sulfuric acid and water can coexist. Sulfuric acid (H2SO4) vapor is produced photochemically from SO3 and water vapor, with more H2SO4 being produced at higher altitudes because of the higher flux of UV radiation.
There is a precedent for spores acting as CCN. Aerial bacteria on Earth act as CCN for ice nucleation (Morris et al., 2004; Creamean et al., 2013). The cells have icenucleating proteins to catalyze the formation of ice nuclei (IN) from liquid (Pandey et al., 2016). Although aerial bacteria are suspected to act as CCN for vapor condensation to liquid based on lab experiments (Bauer et al., 2003), there is not yet any definitive in situ evidence.
The Venusian spores are likely to have a hydrophilic and hygroscopic exterior so they can attract and absorb both sulfuric acid vapor and water vapor. We note that terrestrial bacteria capture water by using hygroscopic biosurfactant polymers. Many microbial polysaccharides and amphipathic lipopeptides, such as syringafactin, from Pseudomonas syringae have highly hygroscopic properties and are instrumental in reducing the water stress of microorganisms (Burch et al., 2014). Once self-encased within a H2SO4 and H2O cloud droplet, the Venusian spore would become solvated, eventually leading to the revival and full physiological activation of the cell. On Earth, spores reactivate when the environmental conditions become favorable for active metabolism and growth. The revival of spores occurs on rehydration and subsequent swelling and the reactivation of metabolic activity (Sella et al., 2014).
We envision that the Venusian spores only constitute a small fraction of CCN, not large enough to affect prior CCN concepts and calculations. The CCN have previously been suggested to be photochemically generated polysulfur compounds (Toon et al., 1982, 1984; Imamura and Hashimoto, 2001). This is a problematic suggestion, because reduced polysulfur solids are likely to be hydrophobic and therefore not able to act as a CCN (Young, 1983; Petrova, 2018). Both meteoritic dust and FeCl3 upwelled from the surface have also been proposed as CCN (Turco et al., 1983; Krasnopolsky, 1985, 2017; Gao et al., 2014). Once nucleation occurs, the initial growth through condensation is rapid, with a timescale on the order of seconds (James et al., 1997).
3.4. Step 4: The cellular life-form lives in the droplet for months to years, depending on the path of the droplet—during this time, the droplet grows by coagulation
Once in the Venus lower clouds the droplets grow and circulate around the atmosphere. The particles will collide, and each collision for liquid particles results in coagulation, leading to further droplet growth. Coagulation timescales [Eq. (3)] are on the order of days to months in the temperate cloud layers (Fig. 3) and particles may grow to sizes greater than 1 mm. Note that at the same time, zonal flow or Hadley cell motions can carry the droplets around the planet with a timescale of days to months (Schubert et al., 1980), but this is not relevant to particle growth.
Once the droplet reaches a large enough size (*1 mm), the cell residing inside the droplet has room to grow and divide. The timescales on which droplets persist in the habitable layer depend on particle size and altitude and are controlled by droplet growth by coagulation and sedimentation due to gravity (Fig. 3). The range can be hours to months to years.
The critical motivating fact for our life cycle description is that droplets will continue to grow by coagulation until the sedimentation timescale becomes shorter than the coagulation timescale, and the particles fall rapidly into deeper, hotter layers of the atmosphere. (Falling much faster than the diffusive transport.) A key question is then: Do the microbes have enough time to metabolize and divide before their droplet home falls to an altitude where they must form spores to survive? The answer is yes. For example, a 3 mmradius particle in the Venusian lower clouds persists for about 6 months, which should be more than enough time for cellular life to germinate from the spore, metabolize, grow, and divide within the same droplet.
Another valid question is whether the droplets in Venus’ temperate cloud decks provide enough habitable space. We argue yes. The most numerous droplets in the Venusian middle and lower clouds are 2 mm in diameter (1 mm in radius) (Fig. 2). As an example, majority of free-living soil bacteria and archaea have a cell diameter smaller than 0.5 mm, with some as small as 0.2 mm diameter (Hahn, 2004; Portillo et al., 2013). The smallest free-living cell size on Earth is *0.2 mm diameter (Luef et al., 2015), leaving a 2 mm droplet diameter (1 mm droplet radius) enough room to accommodate a few cells. Cloud droplets larger than the common 2 mm diameter could contain relatively large microbial communities (droplets on the order of 10 mm diameter or larger could host dozens of cells). The large volume could enhance promotion of cell division.
The small size of some species of microorganisms does not mean that they are biochemically simple, or primitive from the evolutionary standpoint. For example, the smallest known free-living photosynthetic organism is the prokaryote Prochlorococcus, which is 0.5 to 0.7 mm in diameter (Chisholm et al., 1988; Biller et al., 2015).
Although phosphorus is not believed to be a limiting element in Venus’ clouds (Section 2.4), one can envision similar, analogous approaches for other elements as well.
To close out this part of the life cycle, there are two additional peripheral issues. Some particles might updraft up out of the habitable part of the clouds (above 60 km) by being trapped in the Hadley cell flow or by upward diffusion. We note, however, that surviving freezing temperatures in the Venus upper clouds is less challenging than surviving the scorching heat of the lower altitudes. Synthesis of a variety of cryoprotectants is a common strategy employed by life on Earth, including aerial bacteria in Earth’s atmosphere, to mitigate extreme cold (Amato et al., 2019). Life might even adapt to higher altitude layers where the temperature falls significantly below 0C. Similar to the temperature, the concentration of sulfuric acid in cloud droplets changes drastically with altitude. As sulfuric acid remains liquid over a wide-range of temperatures and pressures (Gable et al., 1950; Ohtake, 1993), even cloud droplets residing in the higher cloud decks could remain liquid, where the temperatures and pressures are low (e.g., at 60 km the temperature falls below 0C and pressure falls below 0.5 bar). Life could adapt to temperatures as low as -20C. In fact, surviving in low temperatures is likely much easier to achieve than in high temperature regimes. Earth bacteria routinely survive freezing. Moreover, some obligatory psychrophiles such as Colwellia psychrerythraea 34H are commonly found in sea ice with liquid brine temperatures as low as -35C (Maykut and Untersteiner, 1986), have active motile behavior in temperatures as low as -10C ( Junge et al., 2003), and actively reproduce in temperatures as low as -5C (Huston, 2004). Any particles at high altitudes will eventually return to the habitable layer via the Hadley cell flow, eddy diffusion, or sedimentation. However, at high altitudes high UV irradiation could be destructive if the microbes do not have a strongly UV protective layer (c.f. Section 2.5).
A second peripheral issue is that of droplet fragmentation. If fragmentation could occur, many droplets could avoid growing to sizes with large corresponding sedimentation velocities. More importantly, fragmentation would increase the microbial population in the habitable layer by creating new droplet habitats, some already populated with microbes from prior cell division. By populating the aerial biosphere by fragmentation, the lower haze depot would not be needed.
3.5. Step 5: The bacteria settle down out of the clouds as the droplet reaches a maximum size that can stay aloft against gravity—the decreasing liquid activity triggers cell division and sporulation
Venusian life, trapped living inside of liquid droplets, must adapt to the eventual downward droplet migration to the lower, hotter parts of the Venusian atmosphere. Temperatures during the downward fall increase, and this would result in a gradual loss of liquid activity. In other words, the droplets begin to evaporate, and conditions become inhospitable for physiologically active cells. We hypothesize that in response to degrading conditions (high temperature and low liquid activity), microbes begin metabolic preparations for sporulation and deposition of desiccated spore cells. Desiccated to very small sizes, the spores remain in the lower haze, dormant until upward eddy diffusion caused by mixing induced by gravity waves brings them back to the habitable layer.
Sporulation is the ability of various organisms on Earth to form small, desiccated, and metabolically inactive cells, called endospores, or more generally spores (Setlow, 2006). Sporulation is an adaptation to detrimental environmental conditions that aims at preserving the genetic material of the cell when the surrounding environment is inhospitable or lethal for metabolically active cells. The triggers for sporulation on Earth are stress factors, including dehydration, nutrient limitation, and high cell density (Setlow, 2006; Hutchison et al., 2016). Sporulation allows microorganisms to wait out unfavorable conditions and persist in a dormant state until environmental conditions become favorable again. On Earth, bacterial endospores are superbly resistant to high and low temperatures, high and low pressures, desiccation, radiation, and toxic chemicals (Nicholson et al., 2000). We hypothesize that an analogous process to Earth bacterial sporulation could happen as an essential step during the life cycle of Venusian life. Although sporulation of Earth organisms is often an occasional event triggered as a last resort (Hutchison et al., 2016), as a response to unpredictable environmental conditions, on Venus periodical sporulation events are crucial for microbes’ long-term survival.
The final step in our propsed Venusian life cycle is when the dessicated spores settle out into the lower haze layer. As the spores lose liquid and become less massive, their downward settling times slow further. The individual cells must have a coating that prevents cells clumping together as they dry out, such that each individual spore is a single microbial cell. This critical step is the only way to sustain stable numbers of cells in the cloud decks, as it is the only step in the life cycle where the number of distinct atmospheric particles that contain cells can increase (the number of cells increases during the growth phase in the clouds, but the number of particles containing cells remains the same because the cells all remain in one droplet). For example, fungal, bryophyte, and other spores produced in a dense clump in a sporangium (or equivalent) scatter on release because of the properties of the spore-forming body (Sundberg and Rydin, 1998). Each spore remains dormant until mixing brings it back to the beginning of the life cycle (Section 3.2).
Spores could remain viable in the Venusian atmosphere lower haze layer for long periods, based on analogy with Earth life. On Earth, some bacterial spores can survive in extremely harsh conditions for many thousands of years (Nicholson et al., 2000; Paul et al., 2019). The generation times of some subseafloor sediment bacteria has been estimated to be thousands of centuries (Parkes et al., 2000). More relevant are spores that are viable but have been dormant for thousands of years (Christner et al., 2000; Aouizerat et al., 2019). The currently held, although highly controversial, record for the longest time spent in suspended animation belongs to the spores of the species of Bacillus sealed in a salt crystal that formed 250 Mya ago (Vreeland et al., 2000). Although so far only one such extremely old example is known and the study is controversial as the age of the isolated bacterium is frequently questioned (Maughan et al., 2002), it suggests that Earth-based bacterial spores may be capable of survival in metabolically inactive states for at least several million years (Cano and Borucki, 1995; Meng et al., 2015). Bacteria and archaea are not the only organisms capable of surviving prolonged periods of harsh environmental conditions. Some complex, multicellular organisms (e.g., tardigrades) can remain in suspended animation for several years (Guidetti and JoE` nsson, 2002), as can the dormant eggs of killifish Nothobranchius, which can survive as dried eggs for more than three years and be hatched successfully (Cellerino et al., 2016).